A. Nitrite (NO2) and nitrate (NO3)
Nitrite has the properties toxic to fish. In the blood which contains nitrite reacts with hemoglobin to form methemoglobin as brown blood disease.
Nitrite is formed from the reduction of nitrate by anaerobic bacteria in the bottom waters. In the waters of nitrites can be toxic when concentrations greater than 5 mg / l NO2 – N.
To cope with the level of nitrite poisoning can be added calcium and chloride in these waters.
The process of nitrogen compounds in the water are as follows:
At atmospheric N2 + 3 H2 -> 2 NH3
In the waters of NH3 + H2O -> NH4 + + OH
NH 4 + 4 + 3 O2 -> 2 NO2 + 4 H + + 2 H2O
2 NO2 + O2 -> 2 NO3
NH3 + HNO3 -> NH4NO3
NH4NO3 + O2 -> 2 NO 3 + 2 H + + H2O
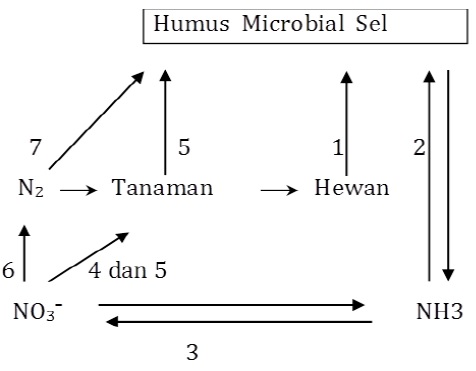
Information;
1- ammonification (formation of ammonia)
2- mineralization (mineralization)
3- Nitrification (the formation of nitrate)
4- Reduction of nitrate
5- Precipitation
6- Denitrification (nitrate solution)
7- N2 Fixation
Ammonia concentration can be lowered by:
1. Improve the aerator.
2. Stop feeding or reduce the amount of feed given.
3. Check the balance of microbiology.
4. When ammonia increased by 0.1 ppm, 10% and make the turn when ammonia to 1.0 ppm, perform a 25% water change. Do not use chlorinated water.
5. Move the fish when ammonia is> 2.5 ppm.
6. Repeating examinations every 12-24 hours
7. Lowering the pH, but not to below 6
B. Plankton
An abundance of plankton which consisted of phytoplankton and zooplankton are needed to determine the fertility of a body of water that will be used in cultivation. Plankton as a low-level aquatic organisms floating in the water in a relatively long time to follow the movement of water. Plankton is generally very sensitive to changes in their environment (temperature, pH, salinity, water movement, sunlight etc.) either to accelerate the development or deadly.
Based on its size, plankton can be distinguished as follows:
1. Macroplankton (they can be seen with the naked eye / regular / without the help of a microscope).
2. Netplankton or mesoplankton (which still can be filtered by a plankton net eyes netnya 0.03 to 0.04 mm).
3. Nannoplankton or microplankton (can get away with a plankton net above).
Based on the place of life and the endemic area, plankton may be:
1. Limnoplankton (freshwater plankton / lake).
2. Haliplankton (live in saltwater)
3. Hypalmyroplankton (Special live in brackish water)
4. Heleoplankton (Special live in ponds)
5. Petamoplankton or rheoplankton (live in fresh water, rivers).
C. Salinity
Water salinity is the total concentration of ions contained in the water. Understanding salinity water which is very easy to understand is the amount of salt content contained in a body of water. This is because the water’s salinity is an overview of the total solids in the water after all carbonates are converted into oxides, all bromide and iodide was replaced by chloride and all organic material has been oxidized.
Understanding salinity water the other is the number of all kinds of salt contained in 1000 g of water sample. Salts that exist in brackish water or seawater is generally Na, Cl, NaCl, MgSO4 that cause a bitter taste in seawater, KNO3 and others. Water salinity can be measured using a device called a refractometer or salinometer (Water Salinity Meter).
Units for the measurement of water salinity are units of grams per kilogram (ppt) or PROMIL (° / оо). Value salinity water for fresh water is usually between 0-5 ppt (salinity Tawar), brackish waters usually ranges between 6-29 ppt (salinity brackish water) and marine waters ranging between 30-35 ppt. (Sea water salinity).