Density of water at different places and times will be different. This difference, although very small, but very important influence on the organism in air.Pengaruh density of the living organisms in the water is the ability of the water to float organisms and other objects. Density is influenced by:
1. Pressure (pressure).
2. Levels of dissolved salts in the water.
3. Salinity suspended in the water.
4. Temperature.
a) The effect of pressure and a certain substance to the density of water
The hydrostatic pressure is a factor that is important for the environment in marine organisms. The deeper the water the higher the pressure hidrostatiknya. Every down as deep as 10 meters, hydrostatic pressure increases by 1 kg / square centimeter or 1 atmosphere. It is conceivable that at a depth of 7,000 meters or hadal region where every animal that lives in the depths must be able to face considerable pressure that is equal to approximately 700 atmosphere.
An enormous pressure. It can be ascertained that the life at that depth is already accustomed to the face of extreme circumstances. Effect of pressure on the density of water is at a higher pressure, the maximum density is reduced. Rise 10 atmospheres (about kira100 m below the surface) temperature of maximum density of water is reduced by approximately 0,1ºC. Furthermore, the influence of certain elements such as dissolved salts can lower the temperature of maximum density of water.
Decrease the temperature by salt amounted 0,2ºC for every 1% increase in salinity. So in the sea that have 35% salinity, temperature of the density maximum is -3,52ºC and this will not be achieved in the liquid phase at normal pressure.
b) The effect of salinity on the density of water
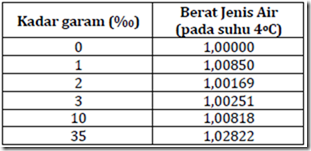
Water density increases with increasing salinity. In Table 1 is an overview of changes in the density of water in relation to the levels of salt in the water. This is because not all waters contain high levels of dissolved material (suspended material) are the same.
Table 1. Effect of salinity (dissolved substance) terhadapberat types of water.
c) The effect of temperature on the density of water
Changes in the density of water caused by temperature changes is important. Water has the properties that its density does not increase with decreasing temperature, but reaches a maximum value at a temperature of 4 ° C (exactly 3,94ºC). After reaching the maximum value at a temperature of 4 ° C, then decreased gradually and just in time freezes density decreased drastically.
Water has special properties between liquids because the molecules tend to be strong to form a group (aggregation) because of its electrical properties are highly dependent on temperature.
Effect of temperature on the water molecules caused a liquid expansion, which in turn gives a graph of temperature and density anomalies. The nature of the anomaly of water is essential for life aquatic organisms in the winter. This is because the water just freezes on top, while the bottom of the water does not freeze, and the temperature is only slightly below 4 ° C. So animals and plants that are under a layer of ice affected the smallest temperature changes than in the mainland.
Therefore, the temperature change did not significantly affect animal and plant life under the ice sheet.
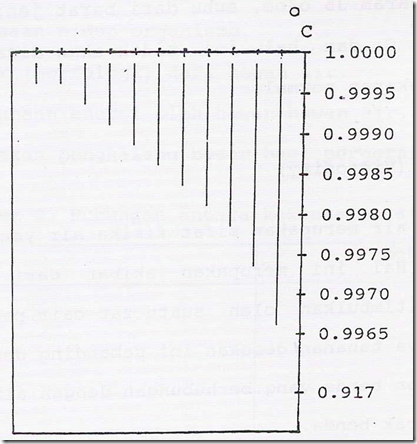
Figure 14. Effect of temperature on the density of water
Regardless of the nature of the water anomaly, the difference in density of water are small due to the influence of temperature change is very important for events in the water. In other words, different types of water would affect the process of life in the waters.