Decreased levels of oxygen.
Basically the process of reduction of oxygen in the water caused by the process of chemistry, physics and biology, namely:
The process of breathing (respiration) either by animals or plants.
The process of decomposition (decomposition) of organic material.
Basic waters are reducing. The basis of these waters can only be covered by only anaerobic bacteria, which can lead to results that are reducing demolition such as methane, sulfide acid and so on. When substances are gaseous rise to the top, the water that passes this gas dissolves while releasing a portion of the oxygen it contains. As a result, more and more water oxygen deficiency.
The level of gas saturation in water sepertikarbondioksida.
The process of evaporation (evaporation) in the summer.
ground water infiltration into the bottom waters
The solubility of oxygen
The solubility of oxygen in water is mainly influenced by temperature. The solubility of oxygen gas at a low temperature relatively higher. The relationship between the temperature of the oxygen solubility in water can be seen in Table 5.
Table 5. The solubility of oxygen at different temperatures
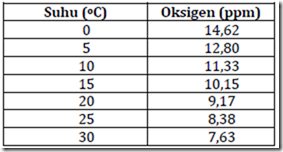
The oxygen solubility applies to fresh water, while the solubility of oxygen in the sea water is relatively lower 1-5 ppm of the above mentioned figures because of the influence of salinity (kadargaram). Salinity affects water solubility of gases. Oxygen solubility is very important because it determines the amount (concentration) of oxygen dissolved in the water. The amount of oxygen in the water at a very decisive aquatic life aquatic organisms.
The limits of tolerance of the organism to oxygen levels depending on the type of the organisms in the water. In general, the minimum threshold levels of oxygen that supports the life of aquatic organisms is 3-5 ppm. In addition to the process of respiration, oxygen also affect the lives of other organisms, namely:
1. Increase the appetite of fish or other aquatic organisms.
2. Affect the health of the fish, which are at the limit of 12 ppm will cause a disease caused by gas bubbles (gas bubble diseases)
3. Affect the physiological function and slow growth of fish, it can even cause death
4. Influencing the process of decomposition of organic matter and overhaul the existing bottom of pool
The concentration of dissolved oxygen in waters fluctuated during the day and night (24 hours). The lowest concentration occurs at dawn (early morning) and then increased during the day and dropping back at night. Differences in the concentration of dissolved oxygen is highest in waters that have a high density of plankton and vice versa. The solubility of oxygen in water is influenced by several factors such as temperature, salinity (salinity) water, surface water movement of water, the area of open water surface, atmospheric pressure and percentage of oxygen around it.
When at the same temperature the dissolved oxygen concentration is equal to the amount of dissolved oxygen in the water, then the water can be said already saturated with dissolved oxygen. If the water contains more dissolved oxygen than it should be at a certain temperature, it means that the oxygen in the water is already supersaturated (super saturation).