It has been explained above that for whatever reason, some of the electrons can leave the atom (electron is called a free electron). If the atom loses electrons are free, it turns into a positive ion. Instead, it would be a negative ion if he accepts free electrons. These ions are unstable and tend to find a trailer to bind to other atoms. Included here is an electrolyte (a substance that transports liquid flow), foundries (eg aluminum smelting) and gas ionization. As a charge carrier in this case is the positive ions and negative ions. Commonly referred to as the ion current.
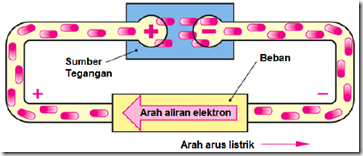
A solution can conduct electricity which is characterized by flaring lights, the electrolyte solution if the substance is able to conduct electricity. Why electrolyte substance can conduct electricity? It is closely related to the ions generated by the electrolyte solution (both positive and negative). A substance can conduct electricity because the compounds have the ions move freely in the solution. ions is what will be the conductor. The more ions produced the better the solution conduct electricity.
Electric current (ion current) in an electrolyte, smelting or gas ionization is a directional movement of ions of materials / liquids. In this case as well as the transfer of materials / substances.
Not a conductor (Insulator)
Electrical insulators are materials that can not or difficult to shift electric charge. In the insulating material tightly bound valence electrons in atoms. These materials are used in electronic equipment as an insulator, or inhibiting the flow of electric current. Isolator also useful as a load-bearing or separation between the conductors without making the currents flowing out or only between the conductors. The term is also used to name the tool used to support electric transmission cables on electricity poles.

Some materials, such as glass, paper, or Teflon is a very good insulator. Some synthetic materials are still “good enough” is used as a cable insulator. Examples of plastic or rubber. These materials have been selected as the cable insulator because it is more malleable / processed while still blocking the flow of electricity on medium voltage (hundreds, perhaps thousands of volts) and is also included herein vacuum gas (also air) with certain rules.
Materials that have few charge carriers and bound in a separate molecule, called the material is not conductive (insulator).